Do Lithium-Ion Batteries show the Memory Effect?

It has long been known that Nickel-Cadmium and older versions of Nickel–Metal Hydride rechargeable cells show the memory effect, but there is uncertainty of its existence in Lithium based cells.
Memory Effect or Voltage Depression was first observed in a Nickel-Cadmium battery which was being used in a satellite. Some of these were the Explorer 6 in 1959 that took the first photos of the Earth, the Ariel 1 in 1962 that was the first in low earth orbit and the Syncom/2 in 1963 that was the first in Geo stationary orbit [1]. It was observed that due to the orbit of the satellite around the Earth, it received a charge for a specific amount of time and discharged to a specific capacity (lets say X%) when away from the sun. This cycle repeated over many months caused a memory effect in the Ni-Cd battery, which made it act like X% was its undervoltage floor.

On further investigation, it was found that the memory effect was caused by changes in the negative or Cadmium plate. Charging involves the conversion of Cd(OH) to Cadmium metal.
Memory can be attributed to changes in the negative or cadmium plate. Recall that charging involves converting CD(OH) to Cd metal. Unde moderate charging currents, the Cadmium metal that is deposited is in the form of small crystals. However, with time in high energy regions these crystals join together to form larger crystals. These larger crystals are harder to dissolve during high current discharge which leads to high internal resistance and the memory effect [3].
So to avoid the memory effect, we need to avoid forming large Cadmium crystals. But as stated before, due to the high energy environment inside the battery and given time, the small crystals join together to form larger ones governed by the laws of thermodynamics. To break the larger crystals, the battery needs a moderate current discharge and charge cycle. This is why on repeated partial discharges, without a full discharge, charge cycle the memory effect is observed.
In Lithium-ion batteries, there is a different type of memory effect that was observed in the LiFePO4 active material, which is found in the LFP type battery (Lithium Iron Phosphate) [4].

There are differences in the memory effect observed in LiFePO4 vs the one previously discussed in Ni-Cd. In Nickel Cadmium, the memory effect is caused by repeated discharges to a partial percentage without a full charge / discharge cycle. In Lithium Iron Phosphate however, a memory effect is seen after the first partial charge, discharge cycle. In the plot shown above, the blue graph represents the first charge, discharge cycle which is termed as the memory writing cycle. The second cycle shown in red is a full charge, discharge cycle, but the existence of a memory effect can be seen in it. The small bump in voltage in the charge cycle of the second cycle (red graph) is based on the memory of the first partial cycle (blue graph). The third cycle full charge, discharge cycle shown in black does not exhibit the memory effect.

The consequence of the memory effect is on voltage measurement of the battery, which in turn effects algorithms such as the State of Charge that rely on accurate voltage readings. An intelligent Battery Management System should store the history of the previous charge, discharge cycle to account for the memory effect it will have on the next cycle.
Higher charge, discharge rates in the partial cycle cause a more pronounced memory bump in the second full cycle. Also, multiple partial cycles cause a larger memory bump in the second full cycle. Therefore, more accurate SOC reading based on voltage, the Battery Management System should hold the history of the last few partial cycles storing at least the % of partial charge / discharge, the charge / discharge rate and number of partial cycles. This will help in distinguishing a bump in voltage caused due to memory as opposed to actual voltage changes.
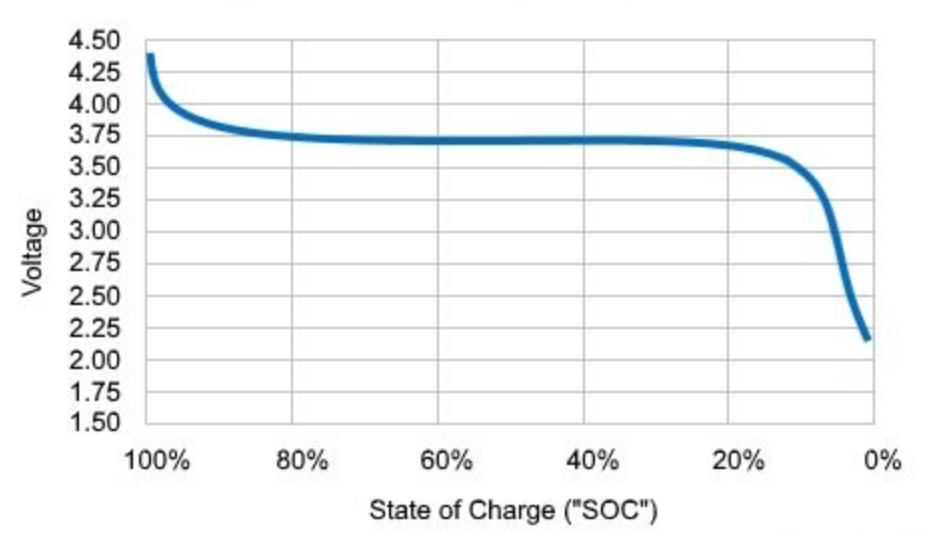
The consequences of a memory effect are higher with battery chemistry that show a flatter voltage curve, as a small change in voltage corresponds to a large change in SOC. The current Lithium-Ion chemistries that exhibit a memory effect as shown above are LFP (LiFePO4) and LTO (Li4Ti5O12) [4], although finding a similar behaviour in more chemistries is contingent on testing.
References, further reading
Get started with our free 10 day course on batteries delivered straight to your email inbox!

